Critical Medical Information Store (CMIS) in Singapore
Critical Medical Information Store allows two-way information sharing of information on reported drug and food allergies. between the EMR systems of national healthcare providers.
Currently, the majority of the Adverse Events reports come through CMIS. The new form is created to encourage AE reporting from healthcare professionals without access to CMIS, as well as those who for specific reasons, prefer to report some AEs outside of the CMIS system. This is in response to the valuable feedback gathered from focus group discussions with healthcare professionals.
Why Should I report adverse events?
When a drug is newly marketed, its safety profile is generally limited to that identified in clinical trials, where evidence is generated based on a small group of individuals who meet specified inclusion and exclusion criteria. Rare AEs also known as adverse events may only surface when many more patients with varying medical conditions get exposed to the drug after it is approved for use in the market. With each AE report received, the safety profile of the drug becomes clearer. This allows HSA to better understand the risk factors of the AEs related to each drug. Such information is valuable for safety monitoring and facilitates prompt detection of potential safety signals, allowing HSA to take appropriate action in a timely manner.
Where does the report go?
Adverse Events reports are submitted through the CMIS e-services and will be reflected on patients’ medical records, then AE reports are received by the HSA.
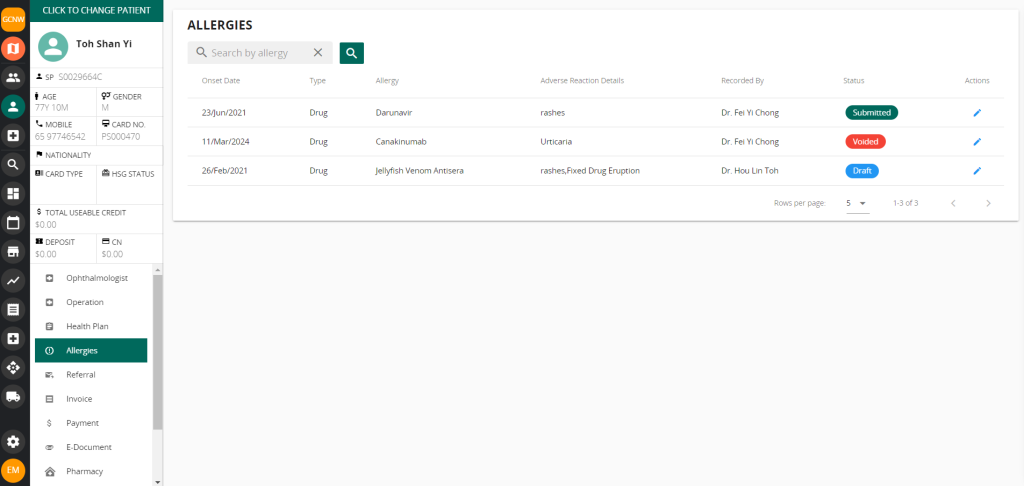
Vanda CMS for Critical Medical Information Store
Using Vanda Clinic Management System (CMS), you can easily send adverse reaction details through the allergy module using NEHR.